Timing is Everything: When to Consider cGMP
Current good manufacturing practice (cGMP) is central to drug manufacturing, helping to produce chemicals and other raw materials in accordance with specific controls and quality systems. Despite cGMPs significant impact on drug quality and consistency, a recent study1 indicated that only about half of biopharmaceutical developers use cGMP chemicals in early-stage clinical trials.
Given the rapid development of complex biologics and the ever-present pressure to advance to production, the consequences of introducing a non-conforming raw material can be catastrophic.
Often, research use only (RUO) raw materials are used in the early stages. RUO materials are not subject to the same rigorous manufacturing and quality controls as cGMP raw materials. For this reason, variables that could be inadvertently introduced by the use of RUO chemicals could result in rework and potential rejection of entire lots of finished drug product.
However, by using cGMP raw materials earlier in the drug development process, manufacturers can minimize these risks and accelerate their development milestones.
A Smooth Transition
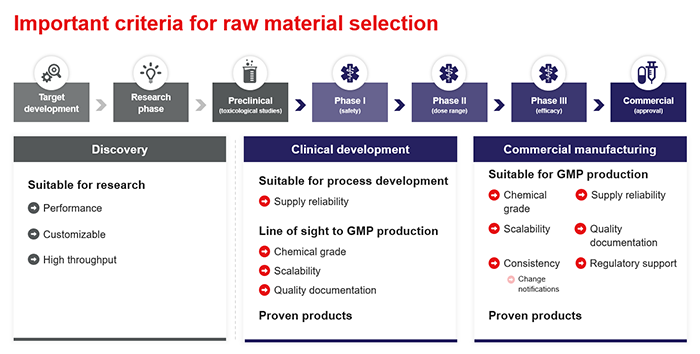
Chemicals manufactured to the rigorous standards of cGMP carry the expectation of high quality. They are sold with comprehensive certification documenting the conformance of each manufactured lot to published standards. The International Council of Harmonisation (ICH) and many other regulatory governing bodies generally stipulate that cGMP-manufactured materials and suppliers be introduced after the completion of preclinical trials.
However, earlier adoption of cGMP chemicals can help make the transition from research phase to clinical production seamless. Doing so more closely simulates the real conditions where the drug will be used in first-in-human (FIH) trials. On the other hand, changing the chemical grade, specification, or supplier when moving into FIH trials requires added validation and qualification, which is time- and resource-intensive. Planning for these compliance and quality standards earlier in the process can greatly help to minimize cost and complexity.
Expect the Unexpected
Competition for vital raw materials has reached unprecedented levels. Though the disruptions that arise from spikes in demand can certainly exacerbate these issues, shortages have been a challenge for quite some time. Assessing supply availability as well as the quality of your chemicals, even for RUO, and establishing relationships with trusted suppliers can go a long way toward mitigating supply disruptions that may arise.
As a related exercise, once your data supports a move to clinical trials, it is worth seeking out higher-quality chemicals from your suppliers. This “pre-work” can ease your way in later stages, as the need to move quickly means taking time to organize materials and document processes, as well as plan for the transition. Better inputs make for better outputs.
Plan for Success
A quality supplier will help you reduce risk, as well as eliminate waste and inefficiencies in the supply of your chemicals. Bringing cGMP into your workflow sooner can help free your teams to focus on what they do best: creating new, potentially life-saving medicines. Such a supplier will have a dedicated team, experienced in cGMP chemical supply, to support you from the early clinical stages all the way through to commercialization, and will be capable of bringing uncommon visibility to your supply chain and transparency to your production planning.
Thermo Fisher Scientific™ offers cGMP chemical distribution and supply chain experience to help customers avoid many scale-up headaches. Through our global relationships with manufacturers and suppliers, our services not only streamline pre-production processes but also keep production moving in compliance with quality and regulatory standards, helping you approach every step of your biomanufacturing workflow with peace of mind.
References
1 Derived from Thermo Scientific Production Chemicals and Services reports and studies, https://www.thermofisher.com/us/en/home/life-science/bioproduction/production-chemicals-services.html

