Learn More
Invitrogen™ Enfortumab Recombinant Monoclonal Antibody
Recombinant Monoclonal Antibody
Brand: Invitrogen™ MA558358
Description
For reconstitution, add sterile, distilled water to achieve a final antibody concentration of 1 mg/mL. Gently shake to solubilize the protein completely. Do not vortex. Reconstituted products should be stored at -80 °.
Enfortumab vedotin is an antibody-pharmaceutical conjugate used in the treatment of patients with advanced, treatment-resistant urothelial cancers. It is comprised of a fully human monoclonal antibody targeted against Nectin-4 and a microtubule-disrupting chemotherapeutic agent, monomethyl auristatin E (MMAE), joined by a protease-cleavable link. It is similar to brentuximab vedotin, another antibody conjugated with MMAE that targets CD-30 instead of Nectin-4. The clinical development of enfortumab vedotin was the result of a collaboration between Astellas Pharma and Seattle Genetics and it was first approved for use in the United States in December 2019 under the brand name PadcevTM.
Specifications
| Enfortumab | |
| Recombinant Monoclonal | |
| Unconjugated | |
| AGS-22C3; AGS-22CE; AGS-22M; AGS-22M6E; AGSM6 | |
| Protein A | |
| RUO | |
| Human | |
| Antibody | |
| IgG1 κ |
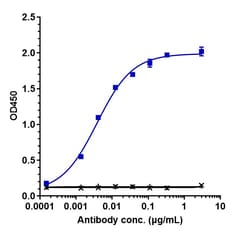
| ELISA, Functional Assay | |
| 1 mg/mL | |
| 25mM histidine with 8% sucrose, 0.01% Tween 80 and no preservative; pH 6.2 | |
| Human | |
| 1 mg | |
| Primary | |
| -20°C, Avoid Freeze/Thaw Cycles | |
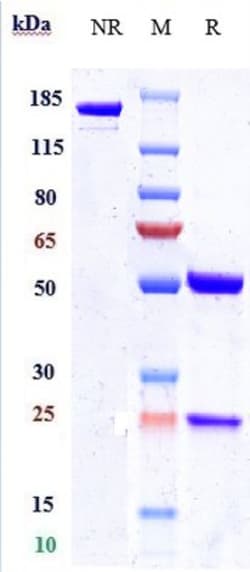
| Lyophilized |
Your input is important to us. Please complete this form to provide feedback related to the content on this product.