Learn More
Invitrogen™ Romosozumab Recombinant Monoclonal Antibody
Recombinant Monoclonal Antibody
Brand: Invitrogen™ MA558772
Description
For reconstitution, add sterile, distilled water to achieve a final antibody concentration of 1 mg/mL. Gently shake to solubilize the protein completely. Do not vortex. Reconstituted products should be stored at -80 °.
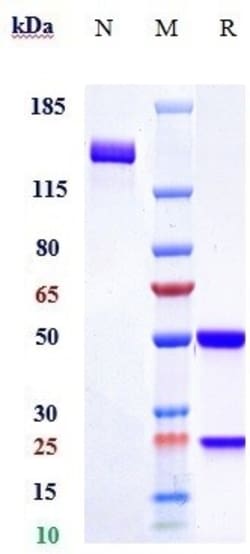
Romosozumab is a humanized monoclonal antibody indicated for the treatment of osteoporosis in postmenopausal women at high risk of fracture and patients who have failed in other treatments or are intolerant to other osteoporosis therapies9. Romosozumab prevents bone resorption and induces the formation of bone though it is associated with an increased risk of cardiac death, heart attack, and stroke in one study7, 8. In a comparison study of post menopausal women with osteoporosis and a past fracture, romosozumab for 12 months followed by alendronic acid for 12 months was superior to alendronic acid alone for 24 months3. Romosozumab also demonstrates a faster and larger increase in bone density than teriparatide4. Romosozumab is marketed in the United States by Amgen under the brand name Evinity7. Romosozumab was granted FDA approval on April 9, 2019.
Specifications
| Romosozumab Humanized | |
| Recombinant Monoclonal | |
| Unconjugated | |
| 785A070802; AMG 785; CDP-7851; romosozumab-aqqg; sclerostin Ab | |
| Protein A | |
| RUO | |
| Human | |
| Antibody | |
| IgG2SA |
| ELISA, Flow Cytometry, Functional Assay, Surface Plasmon Resonance | |
| 1 mg/mL | |
| 25mM histidine with 8% sucrose, 0.01% Tween 80 and no preservative; pH 6.2 | |
| Human | |
| 1 mg | |
| Primary | |
| -20°C, Avoid Freeze/Thaw Cycles | |
| Lyophilized |
Your input is important to us. Please complete this form to provide feedback related to the content on this product.